Right Now
Global AI Pathological Diagnosis Platform Market to Grow at 11.8% CAGR, Reaching USD 3.81 Billion by 2031
Global AI Pathological Diagnosis Platform Market to Grow at 11.8% CAGR, Reaching USD 3.81 Billion by 2031
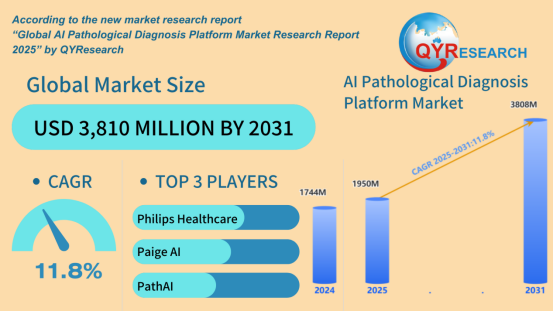
The global market for AI Pathological Diagnosis Platforms is experiencing robust growth, projected to expand at a CAGR of 11.8% from 2025 to 2031, according to the newly released Global AI Pathological Diagnosis Platform Market Research Report 2025 by QYResearch. The market, valued at USD 1.74 billion in 2024, is expected to reach USD 3.81 billion by 2031.
Key Players Driving Innovation
Leading companies in this market include:
· Philips Healthcare
· Roche Diagnostics
· Siemens Healthineers
· GE HealthCare
· Paige AI
· PathAI
· Proscia
· Aiforia Technologies
· Ibex Medical Analytics
· Agfa-Gevaert Group
· Indica Labs
· Deep Bio Inc.
· Visiopharm
· Techcyte
· Hologic Inc.
· Nucleai
· Sectra AB
· OptraSCAN
· Deciphex
· RadNet Inc.
· Qritive
· PathPresenter
· Sun Microsystems AI Lab
· Pathomation
Market Segmentation
By Type
· Image Recognition-Based AI Pathological Platforms
· Deep Learning-Based AI Pathological Platforms
· Integrated Workflow Pathology Platforms
· Multimodal Diagnostic Platforms
· Others
By Application
· Cancer Screening and Diagnosis
· Tissue and Cellular Pathology Analysis
· Surgical Margin Assessment
· Digital Pathology Data Management
· Others
AI Pathological Diagnosis Platform - Product Models by Company
Philips Healthcare
· Pathology Scanner SG60 & SG300 – High-resolution slide scanners for digital pathology.
· Image Management System – Software for managing and analyzing digital pathology images.
· Open Platform – Interoperable with third-party AI applications.
Roche Diagnostics
· VENTANA DP 200 & DP 600 – Whole slide imaging scanners for diagnostics.
· navify Digital Pathology – AI-integrated workflow software.
· AI Image Analysis Algorithms – Tools for evaluating cancer markers like Ki-67, ER, PR.
Siemens Healthineers
· Syngo Carbon – Imaging and reporting solution enhancing pathology workflows.
GE HealthCare
· AI Innovation Lab – Developing foundation models and AI pathology solutions.
Paige AI
· Foundation Models – AI models for cancer detection and biomarker discovery.
· Paige Alba™ – Supports cancer detection, subtyping, biomarker analysis.
PathAI
· PathExplore – Analyzes tumor microenvironments from H&E images.
· PLUTO – Pathology-Universal Transformer foundation model.
Proscia
· Concentriq® AP-Dx – AI-augmented digital pathology workflow platform.
· Concentriq Embeddings – Pathology foundation models for AI development.
Aiforia Technologies
· Aiforia® Clinical Suite – Detects and quantifies metastases in cancer cases.
· Aiforia® Create – Enables creation of deep learning AI models from image data.
Ibex Medical Analytics
· Galen™ Platform – AI for cancer detection and diagnostic accuracy improvement.
Agfa-Gevaert Group
· Enterprise Imaging – Manages images across vendor-neutral digital pathology systems.
Indica Labs
· HALO® & HALO AI – Platforms for whole slide image evaluation with AI.
· HALO AP® & HALO AP Dx – Digital pathology solutions with clinical clearance.
Deep Bio Inc.
· DeepDx Prostate – AI model for grading prostate cancer.
· Breast PR IHC – Assesses PR receptor status in breast cancer.
Visiopharm
· Oncotopix Discovery – Image analysis for tissue-based research.
· Phenoplex v3 – Multiplex analysis with spatial context features.
Techcyte
· Fusion Platform – Clinical pathology platform with AI tools.
· SureView™ Cervical Cytology System – AI-assisted precancerous lesion detection.
Hologic Inc.
· Genius™ Digital Diagnostics System – Digital cytology system using AI for cervical cancer detection.
· Genius AI Detection – AI tool for identifying breast cancer lesions.
Latest Trends and Developments in 2025
Proscia’s Major Recognition in 2025
In 2025, Proscia was awarded the prestigious MedTech Breakthrough Award for its Concentriq® AP-Dx platform. The platform is recognized for its innovation in transforming digital pathology into a scalable and routine component of clinical diagnostics. Concentriq® AP-Dx is designed for high-throughput laboratories and leverages AI to accelerate workflows, supporting more accurate and efficient cancer diagnosis.
Deciphex Raises €31 Million to Expand Diagnexia and Patholytix
Deciphex, an AI pathology leader based in Ireland, announced a €31 million Series C fundraising round in early 2025. The funding will support global expansion of its flagship platforms—Diagnexia and Patholytix—which provide digital consultation and remote diagnostics. These platforms aim to reduce diagnostic bottlenecks and address the global shortage of pathologists, particularly in North America and the EU.
Harvard’s Foundation Model “CHIEF” Achieves Breakthrough in Cancer Diagnosis
Researchers at Harvard Medical School unveiled CHIEF (Clinical Histopathology Imaging Evaluation Foundation), a groundbreaking AI foundation model trained on over a million unlabelled pathology images. CHIEF demonstrated up to 94% accuracy in diagnosing multiple types of cancer and offers significant advantages in identifying the origin of tumors and predicting genetic mutations, without the need for genomic sequencing.
PathVis Combines AI with Mixed Reality for Pathology Visualization
PathVis, introduced in 2025, represents a new frontier in digital diagnostics by integrating artificial intelligence with mixed reality. Designed for use with devices like the Apple Vision Pro, PathVis enables pathologists to interact with digital slides using gestures, eye-tracking, and voice commands. It features real-time case retrieval and cross-reference capabilities, reducing diagnostic errors and improving user ergonomics.
Emerging Trends Shaping 2025 and Beyond
· Rise of Foundation Models: Like CHIEF, general-purpose AI models are being developed to handle multiple diagnostic tasks across diverse pathology datasets.
· Adoption of Multimodal Interfaces: Tools like PathVis show growing interest in combining visual computing with AI for enhanced diagnosis.
· Global Expansion: Companies such as Deciphex are expanding services globally, reflecting growing demand for AI-driven diagnostic support amid workforce shortages.
· Clinical Integration: Platforms like Proscia's Concentriq® AP-Dx are becoming embedded in hospital and lab infrastructures, showing AI’s transition from R&D to daily clinical use.
These developments signal a transformative leap in how AI is applied in pathology, offering speed, scale, and enhanced accuracy that will define the future of diagnostic medicine.
Regional Outlook
Significant growth is expected across regions:
· North America: Continuous adoption of AI-driven diagnostics across the U.S. and Canada.
· Asia-Pacific: Surge in healthcare digitization in China, Japan, and South Korea.
· Europe: Integration of AI in national health systems like NHS (UK) and HSE (Ireland).
The AI Pathological Diagnosis Platform market is transforming global healthcare, enabling precise, timely, and cost-effective diagnostic services. With continued technological advancement and adoption, the industry is poised to become a foundational element in pathology and oncology diagnostics worldwide by 2031.
Get free Sample report:
Global AI Pathological Diagnosis Platform Market Research Report 2025
https://www.qyresearch.com/reports/4715808/ai-pathological-diagnosis-platform
AI Pathological Diagnosis Platform - Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031
https://www.qyresearch.com/reports/4715805/ai-pathological-diagnosis-platform
Global AI Pathological Diagnosis Platform Sales Market Report, Competitive Analysis and Regional Opportunities 2025-2031
https://www.qyresearch.com/reports/4715797/ai-pathological-diagnosis-platform
Global AI Pathological Diagnosis Platform Market Insights, Forecast to 2031
https://www.qyresearch.com/reports/4715794/ai-pathological-diagnosis-platform
About Us
QYResearch founded in California, USA in 2007. It is a leading global market research and consulting company. With over 18 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 66,000 clients across five continents. Let’s work closely with you and build a bold and better future
Contact Us:
If you have any queries regarding this report or if you would like further information, please contact us:
QY Research Inc.
Add: 17890 Castleton Street Suite 369 City of Industry CA 91748 United States
EN: https://www.qyresearch.com
Email: global@qyresearch.com
Tel: 001-626-842-1666(US)
JP: https://www.qyresearch.co.jp
More Posts


















Report This Post
Please complete the following requested information to flag this post and report abuse, or offensive content. Your report will be reviewed within 24 hours. We will take appropriate action as described in Findit terms of use.


